Cathodic protection or CP, in short, is a method that is used for controlling corrosion of any metal surface. It does so by making those metal surfaces as the cathodes of electrochemical cells. In the process of cathodic protection, the metal that needs to be protected is connected to a sacrificial metal to act as an anode. The metal that acts as an anode in this connection is relatively easily corrodible. Hence it is also called the sacrificial metal and has relatively lesser importance. The sacrificial metal in this process corrodes in place of the metal that we intend to protect. For constructions such as the long and big pipelines, in which inert galvanic cathodic defense is not sufficient, as external DC (Direct Current) power source is applied in order to suffice the provision of electric current.
Vacker KSA supplies a wide range of cathodic protection systems that protects a wider range of metal structures in a diversified environmental condition in Riyadh, Al Khobar, Dammam, Jeddah, etc. Commonly used applications are fuel pipelines, home water heaters, ship/boat hulls, oil well casing and metal strengthening bars in concrete structures. Galvanized steel can be taken as another common example of cathodic protection. In this process zinc is the sacrificial metal which acts as the anode. Zinc is coated on steel so that steel is protected from being rusted. Cathodic protection can also protect some metal surfaces from cracking because of stress corrosion.
History of Cathodic Protection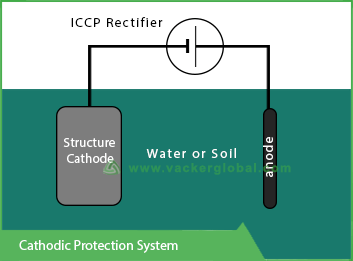
Cathodic protection was primarily portrayed by Sir Humphry Davy. It was back in 1824 AD when he explained the process of cathodic protection in a sequence of papers that were presented in London to the Royal Society. In the primary application of the cathodic protection, irons were used as sacrificial anodes that were connected to the copper sheath. This radically decreased the rate of corrosion of the copper.
Thomas Alwa Edison also experimented on the process of cathodic protection in 1890. His experiment of cathodic protection was on ships. Sadly, he was not successful in his experiments because of lack of sufficient power source and the anode materials.
Types of Cathodic Protections
There are basically two types of cathodic protection systems that Vacker KSA has in offer for its users all over the country. The types of cathodic protections have been detailed as under:
Galvanic Cathodic Protection
In this system, a galvanic anode is attached to the metal surface that is supposed to be vulnerable when exposed to an electrolyte. In this application, the galvanic anode is a more electrochemically active piece of metal. The galvanic anodes are chosen because they have more negative electrode potential that the vulnerable metals.
The electrons flow from anode to cathode which causes the polarization of target metals. Hence both the metals should have a very good electrically conductive contact. There is a difference between the anode and the cathode in connection to electrode potential which is the ultimate force for the current required for cathodic protection.
Vacker KSA supplies a variety in shape and size of galvanic or the sacrificial anode. They are made of alloys using zinc, aluminium and magnesium. ASTM International is responsible for publishing the standards on composition along with manufacturing of galvanic anodes.
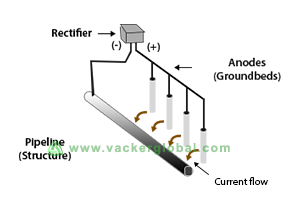
Impressed Current Systems
A simple impressed current system of cathodic protection uses Direct Current (DC) as a source of electric current in order to trigger the electrochemical reaction.
Applications of Cathodic Protection
The applications of Cathodic protection are listed below:
- Pipelines
- Ships and boats
- Marine
- Internal cathodic protection
- Steel in concrete
- Galvanized steel
- Automobiles
Testing of Cathodic Protection
The potential of an electrode is measured with the help of reference electrode. Depending upon the structures, various electrodes are used.
- Copper-copper sulphate electrodes – structures in contact with fresh water and soil.
- Silver/silver chloride/seawater electrodes – seawater applications.
Limitations of Cathodic Protection
Although cathodic protection has a number of significances, there are a few limitations to the use of cathodic protection. Some of them have been briefed as under:
Production of Hydrogen
Production of atomic hydrogen may occur as a side effect if the cathodic protections are applied in an improper manner. Thus produced hydrogen in eventually absorbed by the protected metal, which further leads to hydrogen embrittlement in very hard metals. The hydrogen atoms can react with the metal surface to form hydrogen gas leading to hydrogen embrittlement.
Cathodic Disbanding
The protective coating from the cathode is disbanded because of the formation of hydrogen ions on the surface of the cathode.
Cathodic Shielding
The phenomena of cathodic shielding occur because of the high electric resistivity of the film backings used in various processes. They are used in steel pipelines. The electrolytes are shielded from reaching the destination hence disturbing the efficiency of the entire process.
